Heart
The Heart
Normal Structure
1. Anatomy and Physiology
-
Heart Function: Muscular pump that circulates blood through the body.
-
Weight:
-
Adult Male: 300-350 gm
-
Adult Female: 250-300 gm
-
-
Chambers:
-
Right Atrium and Left Atrium (superior)
-
Right Ventricle and Left Ventricle (inferior and larger)
Septa:
-
Interatrial septum: Thin wall between atria.
-
Interventricular septum: Thick wall between ventricles.
Wall Thickness:
-
Right Ventricle: 0.3–0.5 cm
-
Left Ventricle: 1.3–1.5 cm
2. Blood Flow Pathway
Systemic veins → Right atrium → Right ventricle → Pulmonary arteries → Lungs → Pulmonary veins → Left atrium → Left ventricle → Aorta → Systemic arteries
3. Cardiac Valves
-
Atrioventricular (AV) Valves:
-
Tricuspid valve (right)
-
Mitral/Bicuspid valve (left)
-
-
Semilunar Valves :
-
Pulmonary valve
-
Aortic valve
-
4. Wall Layers of the Heart
-
Epicardium (Visceral Pericardium): Outer covering
-
Myocardium: Muscle layer
-
Endocardium: Inner lining
5. Myocardium Structure
-
Muscle Fibers: Branching, striated (pattern of light and dark bands seen in certain muscle tissues under a microscope. These bands are caused by the regular arrangement of actin and myosin filaments), arranged in parallel.
-
Intercalated Discs: Connections for ion and action potential movement.
-
Mitochondria: Abundant for ATP production.
-
Sarcoplasmic Reticulum: Stores calcium ions.
-
Sarcomere Structure:
-
A-band: Thick myosin filaments
-
I-band: Thin actin filaments
-
Tropomyosin: Covers actin
-
Troponin Complex:
-
Troponin-I, Troponin-T, Troponin-C (regulate contraction)
-
-
6. Conduction System of the Heart
Controls heart rate and rhythm:
-
SA Node (Sinoatrial Node): Pacemaker (Right atrium wall)
-
AV Node (Atrioventricular Node): Top of interventricular septum
-
AV Bundle (Bundle of His): Carries impulse down the septum (facilitating the transmission of electrical impulses from the atrioventricular (AV) node to the ventricles)
-
Bundle Branches: Right and left bundles to ventricles
7. Pericardium
-
Visceral Pericardium (Epicardium): Inner layer
-
Parietal Pericardium: Outer fibrous sac
-
Pericardial Cavity: Space containing 10–30 ml serous fluid (lubricates and protects heart)
8. Endocardium
-
Inner smooth lining of heart chambers, valves, chordae tendineae (fibrous cords that connect the papillary muscles to the tricuspid and mitral valves in the heart), and papillary muscles (pillar-like muscle projections located within the ventricles of the heart).
-
Made of endothelium, connective tissue, and elastic fibers.
9. Heart Valves
-
Thin, translucent structures.
-
Strengthened by collagen and elastic tissue.
-
Covered by endothelium (valvular endocardium).
10. Myocardial Blood Supply
-
Supplied by coronary arteries originating just above the aortic valve.
-
Most blood flow happens during diastole (relaxation phase) ?.
Major Coronary Arteries:
-
Left Anterior Descending Artery:
-
Apex, anterior left ventricle, part of right ventricle, and septum (2/3rd).
-
-
Circumflex Artery:
-
Left atrium and lateral left ventricle.
-
-
Right Coronary Artery:
-
Right atrium, anterior right ventricle, part of left ventricle, and posterior septum (1/3rd).
-
12. Venous Drainage
-
Coronary veins collect blood after oxygen is used.
-
Drain into the coronary sinus.
HEART FAILURE
Definition
Heart failure is a pathophysiological condition where the heart is unable to pump enough blood to meet the metabolic demands of the body.
Etiology (Causes)
1. Intrinsic Pump Failure
These conditions directly impair the contractile function of the heart muscle.
a. Ischemic Heart Disease (IHD)
-
Mechanism:
-
Atherosclerosis in coronary arteries → reduced oxygen supply → myocardial ischemia or infarction.
-
Infarcted myocardium is replaced by non-contractile fibrous tissue.
-
Leads to ventricular remodeling, thinning, dilation → ↓ ejection fraction.
-
-
Type of HF: Systolic heart failure
b. Myocarditis
-
Etiology: Viral (e.g., coxsackievirus B), bacterial, autoimmune, toxins.
-
Mechanism:
-
Inflammation damages myocytes
-
Weakens contractility; can evolve into dilated cardiomyopathy.
-
-
Type of HF: systolic heart failure.
c. Cardiomyopathies
-
Dilated Cardiomyopathy:
-
Thin, stretched ventricles → poor systolic contraction.
-
Cause: Idiopathic, alcohol, genetic, toxins, post-viral.
-
HF Type: Systolic.
-
-
Hypertrophic Cardiomyopathy:
-
Thickened myocardium → impaired filling.
-
Often genetic; causes diastolic dysfunction.
-
-
Restrictive Cardiomyopathy:
-
Stiff walls due to infiltration (amyloidosis).
-
Impaired filling.
-
HF Type: Diastolic.
-
d. Metabolic Disorders (e.g., Beriberi)
-
Beriberi: Thiamine (B1) deficiency.
-
Mechanism:
-
Thiamine needed for ATP generation.
-
Deficiency impairs myocardial energy metabolism → weak contractility.
-
-
HF Type: High-output failure → progresses to systolic HF.
e. Arrhythmias
-
Mechanism:
-
Irregular, rapid contraction → poor ventricular filling and output.
-
-
HF Type: Both systolic and diastolic, depending on duration and severity.
2. Increased Workload on Heart
These conditions overload the heart either by pressure or volume, leading to hypertrophy and eventual failure.
a. Pressure Overload
i. Hypertension (Systemic or Pulmonary)
-
Systemic HTN → Left ventricular hypertrophy → diastolic dysfunction → eventual systolic failure.
-
Pulmonary HTN (e.g., due to COPD) → Right ventricular hypertrophy → right-sided HF.
ii. Valvular Stenosis (Aortic, Mitral, Pulmonary)
-
Mechanism:
-
Obstruction to outflow (aortic/pulmonary).
-
Increases pressure load on ventricles or atria.
-
Long-term hypertrophy → ↓ compliance and eventual failure.
-
-
HF Type: Initially diastolic, progresses to systolic.
iii. Chronic Lung Disease
-
Mechanism:
-
Hypoxia → pulmonary vasoconstriction → ↑ pulmonary artery pressure.
-
Right ventricular pressure overload → hypertrophy → right-sided HF.
-
b. Volume Overload
i. Valvular Insufficiency (Regurgitation)
-
Mechanism:
-
Retrograde flow increases volume.
-
Volume overload leads to dilation → systolic dysfunction.
-
-
Examples: aortic regurgitation.
ii. Anaemia
-
Mechanism:
-
Decreased oxygen-carrying capacity → increased cardiac output demand.
-
Chronic high-output state strains heart.
-
iii. Thyrotoxicosis
-
Mechanism:
-
Increased metabolism and O2 demand.
-
Increases heart rate and contractility → chronic strain.
-
iv. Chronic Hypoxia (e.g., from lung disease)
-
Mechanism:
-
Pulmonary vasoconstriction → right ventricular overload.
-
Leads to right heart failure.
-
3. Impaired Filling of Chambers
These conditions restrict diastolic filling, leading primarily to diastolic heart failure.
a. Cardiac Tamponade
-
Mechanism:
-
Fluid accumulation in pericardial sac → ↑ pericardial pressure.
-
Prevents ventricular filling → ↓ stroke volume and output
-
b. Constrictive Pericarditis
-
Mechanism:
-
Rigid, thickened pericardium (post-infectious, TB) restricts diastolic filling.
-
Leads to elevated venous pressures, ↓ output
-
Types of heart failure
Acute vs Chronic Heart FailureA. Acute Heart Failure
B. Chronic Heart Failure
II. Left-Sided vs Right-Sided Heart FailureA. Left-Sided Heart Failure
B. Right-Sided Heart Failure
| ||
I. Overview of Heart Failure Terminology
II. Forward Heart FailureDefinitionInability of the heart to pump sufficient blood forward into the arterial circulation to meet the metabolic demands of tissues. Pathophysiology
Causes
Clinical Features
III. Backward Heart FailureDefinitionFailure of the heart to effectively eject blood → leads to increased venous pressures behind the affected chamber → congestion in pulmonary or systemic circulation. Pathophysiology
Types
Causes
Clinical FeaturesLeft-Sided Backward Failure:
Right-Sided Backward Failure:
|
Compensatory Mechanisms
-
Cardiac hypertrophy and dilatation
-
Tachycardia due to neurohumoral activation (norepinephrine, ANP, RAAS)
-
Starling’s Law: Dilated heart increases sarcomere length to maintain stroke volume — eventually fails
Cardiac Hypertrophy
-
Definition: ↑ Myocardial size and weight
-
Stimuli: Mechanical stretch, hypoxia, hormonal factors
A. Left Ventricular Hypertrophy Causes:
-
Systemic hypertension, aortic/mitral disease, CAD, coarctation, thyrotoxicosis, anaemia
B. Right Ventricular Hypertrophy Causes:
-
Pulmonary hypertension, tricuspid disease, mitral stenosis, chronic lung disease, left heart failure
Cardiac Dilatation
-
Due to volume overload → elongation of fibres
Causes:
-
Valvular insufficiencies (mitral/aortic/tricuspid/pulmonary)
-
Left-to-right shunts (e.g., VSD)
-
High-output states (thyrotoxicosis, AV shunts)
-
Cardiomyopathies, hypertension
Morphological Features
-
Hypertrophy Types:
-
Concentric: Pure hypertrophy → ↑ wall thickness, ↓ lumen
-
Eccentric: With dilatation → ↑ lumen size
-
-
Gross: Heart weight >500 gm; LV wall >15 mm
-
Microscopy:
-
↑ fibre size
-
Foci of necrosis
-
Relative hypoxia in deep myocardium
-
↑ RNA/DNA ratio, ↑ myofilaments, multiple intercalated discs
-
Summary
-
Left-sided failure: Pulmonary symptoms (dyspnea, fatigue)
-
Right-sided failure: Systemic congestion (oedema, hepatomegaly)
-
CHF = Combination of both sides
-
Long-term compensation leads to hypertrophy, then decompensation
Congenital Heart Disease (CHD)
Definition
-
Structural abnormalities of the heart present at birth.
-
Most common heart disease in infants (~0.5% of newborns); higher in preterm infants (before 37 weeks).
Etiology
-
Largely unknown; multifactorial (genetic + environmental).
-
Risk factors:
-
Maternal rubella infection
-
Drugs and alcohol during pregnancy
-
Fetal injury during gestation (These injuries can disrupt normal cardiac embryogenesis, especially during the first trimester when the heart is forming (3rd to 8th weeks of gestation)
-
Classification of Congenital Heart Diseases
| Group | Examples |
|---|---|
| I. Malpositions | Dextrocardia (± situs inversus) |
| II. Shunts | A. Left-to-Right Shunts (Acyanotic or late cyanotic) VSD, ASD, PDA B. Right-to-Left Shunts (Cyanotic) Tetralogy of Fallot, Transposition of great arteries, Persistent truncus arteriosus, Tricuspid atresia/stenosis |
| III. Obstructions | Coarctation of aorta, Aortic stenosis/atresia, Pulmonary stenosis/atresia |
I. Malpositions of the Heart
Dextrocardia
-
Heart apex points to the right.
-
May be isolated or part of situs inversus.
-
Isolated dextrocardia often linked with severe anomalies like transposition of great vessels.
II. Shunts
A. Left-to-Right Shunts (Acyanotic → Late Cyanosis)
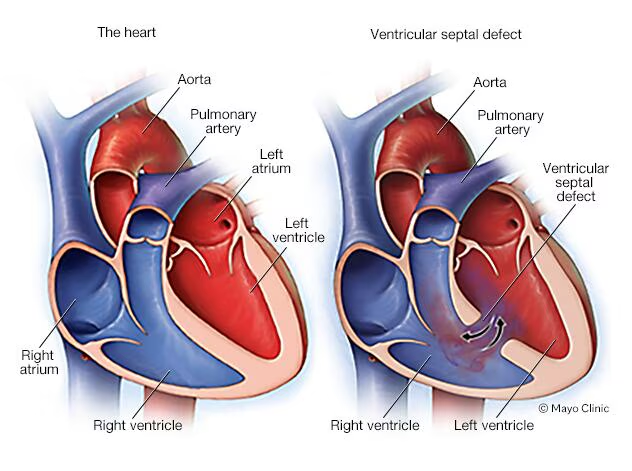
1. Ventricular Septal Defect (VSD)
Most common CHD (30%)
-
Types:
-
Membranous septum (Upper portion of the interventricular septum, near the atrioventricular (AV) node and valves (aortic and tricuspid)) (90%)
-
Subpulmonic (Just below the pulmonary valve), subaortic (Just below the Aortic valve, muscular septum (In the muscular (trabeculated) part of the septum, anywhere from mid to lower septal regions) - 10%
-
Effects:
-
↑ Pulmonary blood flow
-
Right ventricular volume hypertrophy
-
Left atrial and ventricular hypertrophy (In VSD, blood from the left ventricle flows into the right ventricle. This shunted blood is sent to the lungs, oxygenated, and then returns to the left atrium via the pulmonary veins. Result: Extra blood volume enters the LA and LV, increasing their workload and then hypertrophy)
-
Valve changes (tricuspid, pulmonary, mitral, aortic); Left-to-right shunting increases pulmonary blood flow, returning excess volume to the left atrium and ventricle. This causes chamber dilation, especially of the left-sided chambers. As the ventricular and atrial walls stretch, the valve annuli (rings) stretch too.
2. Atrial Septal Defect (ASD)
-
~10% of CHDs
-
Types:
-
Ostium secundum (90%) – fossa ovalis (opening in the fetal heart that allows blood to bypass the lungs, which are not yet functional. It connects the right atrium directly to the left atrium, allowing oxygen-rich blood from the placenta to go directly to the systemic circulation)
-
Ostium primum (5%) – lower septum; may involve mitral valve cleft
-
Sinus venosus (5%) – upper septum
-
Effects:
-
Right atrial and ventricular hypertrophy (the right atrium receives excess blood from both the systemic veins and the left atrium via the shunt, leading to increased volume load. The right atrium dilates and hypertrophies over time. The overloaded right atrium then pumps more blood into the right ventricle, increasing its preload, which causes the right ventricle to hypertrophy)
-
Small left heart chambers and orifices (In Atrial Septal Defect (ASD), blood shunts from the left to the right atrium, reducing the amount of blood reaching the left heart. As a result, the left atrium and ventricle receive less blood, leading to smaller left heart chambers and narrower orifice).
-
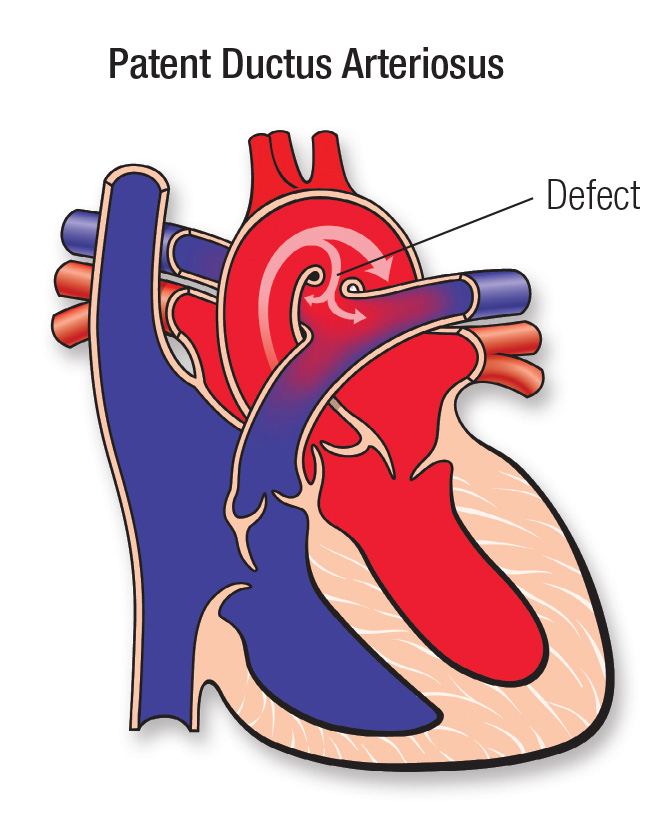
3. Patent Ductus Arteriosus (PDA)
-
Ductus fails to close by 3 months
-
Often isolated (90%), may coexist with other defects
-
Causes: Persistent PGE₂ synthesis
-
Effects:
-
↑ Pulmonary flow (more oxygenated blood from the left side of the heart to flow into the right side (right atrium and right ventricle), which is then pumped to the lungs via the pulmonary artery)
-
Left heart hypertrophy (increased pulmonary blood flow, the left atrium receives more blood than normal. As the left atrium enlarges, the left ventricle also begins to take in excess blood and has to work harder to accommodate the increased volume)
-
Aortic and mitral valve enlargement (increased volume can cause stretching of the mitral valve annulus
-
B. Right-to-Left Shunts (Cyanotic)
1. Tetralogy of Fallot
-
Most common cyanotic CHD (10%)
-
Four components:
-
VSD
-
Overriding aorta
-
Pulmonary stenosis
-
Right ventricular hypertrophy
Forms:
-
Cyanotic: Severe stenosis → R-to-L shunt
-
Acyanotic: Large VSD, mild stenosis → L-to-R shunt
2. Transposition of Great Arteries
-
Regular: Aorta from RV, pulmonary trunk from LV → cyanosis at birth
-
Corrected: Both great arteries and veins transposed → physiological correction
3. Persistent Truncus Arteriosus
-
Single arterial trunk from both ventricles
-
Often with VSD
-
Early cyanosis; poor prognosis
4. Tricuspid Atresia/Stenosis
-
Absence or malformation of tricuspid valve
-
Associated interatrial defect → R-to-L shunt
-
Cyanosis at birth, short survival
III. Obstructive Congenital Heart Diseases
These involve impeded outflow of blood from the heart chambers.
1. Coarctation of the Aorta
-
Narrowing of the aorta, usually near the ductus arteriosus.
-
Types:
-
Preductal (infantile).
-
Postductal (adult-type).
-
-
May present with:
-
Upper limb hypertension.
-
Lower limb hypotension.
-
2. Aortic Stenosis / Atresia
-
Obstruction of left ventricular outflow.
-
May range from valvular narrowing to complete atresia.
-
Leads to:
-
LV hypertrophy.
-
Possible hypoplastic left heart syndrome in severe cases.
-
3. Pulmonary Stenosis / Atresia
-
Obstruction of right ventricular outflow.
-
May occur alone or with other defects (e.g., TOF).
-
Can cause:
-
RV hypertrophy.
-
Summary Chart: Clinical and Pathological Features
| Defect | Shunt Direction | Cyanosis | Key Features |
|---|---|---|---|
| VSD | Left to Right | No (early) | RV, LA, LV hypertrophy |
| ASD | Left to Right | No | RA, RV enlargement |
| PDA | Left to Right | No | Machine-like murmur, bounding pulses |
| Tetralogy of Fallot | Right to Left | Yes (early) | Cyanosis, squatting, boot-shaped heart |
| TGA | Right to Left | Yes (birth) | Requires surgical correction |
| Persistent Truncus Arteriosus | Mixed | Yes (early) | Single trunk, early CHF |
| Tricuspid Atresia | Right to Left | Yes (birth) | Hypoplastic RV, short survival |
| Coarctation of Aorta | None (Obstructive) | No | BP differential in limbs, rib notching |
| Aortic Stenosis | None (Obstructive) | No | LV hypertrophy |
| Pulmonary Stenosis | None (Obstructive) | Possible | RV hypertrophy, may cause cyanosis if severe |
Ischemic Heart Disease (IHD)
-
Definition: IHD is heart damage due to imbalance between oxygen supply and demand to heart muscle.
-
Main cause: Narrowing or blockage of coronary arteries (usually from atherosclerosis).
-
Also called Coronary Artery Disease (CAD).
-
Most common cause of death in developed countries; increasing globally.
Epidemiology
-
More common in men than women (until menopause).
-
By 2020s, predicted to be #1 cause of death worldwide.
Causes of IHD
I. Coronary Atherosclerosis (90%)
-
Major cause of IHD due to "fixed" blockages in coronary arteries.
-
Common sites:
-
Left anterior descending (most common)
-
Right coronary artery
-
Left circumflex artery
-
-
Significant blockage: >75% reduction in lumen.
II. Superadded Changes on Atherosclerosis
-
Plaque rupture or ulceration → triggers clot formation.
-
Coronary thrombosis → causes complete artery blockage.
-
Platelet aggregation & spasm → worsens blockage and reduces blood flow.
III. Non-Atherosclerotic Causes (Less than 10%)
-
Vasospasm (e.g., from drugs or unknown causes)
-
Coronary artery inflammation (e.g., Kawasaki, Takayasu, infections)
-
Embolism (e.g., from infective endocarditis)
-
Blood disorders (e.g., sickle cell, polycythemia)
-
Trauma
-
Aneurysms or tumors compressing the artery
Effects of Myocardial Ischemia
Depends on severity, location, and duration of blood supply interruption.
1. Asymptomatic
-
Minor narrowing; no symptoms
2. Angina Pectoris
-
Chest pain on exertion due to temporary ischemia
3. Myocardial Infarction (MI)
-
Heart attack due to complete blockage
4. Chronic IHD / Ischemic Cardiomyopathy
-
Long-standing poor blood flow → heart muscle damage, fibrosis
5. Sudden Cardiac Death
-
Sudden heart stop, usually from arrhythmia
🔺Acute Coronary Syndromes = MI + Unstable Angina + Sudden Cardiac Death
ANGINA PECTORIS
A clinical syndrome of Ischemic Heart Disease (IHD) caused by transient myocardial ischemia, presenting as paroxysmal chest pain. Common in men over 50 years.
Pain Characteristics:
-
Location: Substernal/precordial
-
Radiation: Left arm, neck, jaw, or right arm
-
Triggered by exertion/emotion
-
Relieved by rest or nitroglycerin
Types of Angina:
| Type | Features |
|---|---|
| Stable (Typical) Angina | Most common; triggered by exertion; due to chronic atherosclerosis; ST depression, no enzyme rise |
| Prinzmetal’s (Variant) | Occurs at rest; due to coronary vasospasm; ST elevation; responds to vasodilators |
| Unstable (Crescendo) | Occurs at rest, more frequent and severe; warning of impending MI; ECG: NSTEMI or STEMI possible |
ACUTE MYOCARDIAL INFARCTION (MI)
Irreversible myocardial necrosis due to prolonged ischemia; the most serious consequence of coronary artery disease.
Epidemiology:
-
Accounts for 10–25% deaths in developed nations
-
5% occur in <40 years (especially with risk factors)
-
More common in males
-
Oestrogen is protective in females (incidence ↑ after menopause)
Etiopathogenesis:
-
Coronary atherosclerosis (>75% narrowing) – major cause (90%)
-
Platelet aggregation → thrombosis
-
Plaque rupture/haemorrhage
-
Non-atherosclerotic causes (10%): Vasospasm, embolism, arteritis
-
Transmural vs Subendocardial infarcts
-
Transmural: Full wall thickness, common (95%), usually due to thrombosis
-
Subendocardial: Inner wall only; due to hypoperfusion without critical stenosis
-
CLASSIFICATION OF INFARCTS
| Classification | Types/Examples |
|---|---|
| Anatomic Site | Anterior, Posterior, Septal, Lateral, Combinations |
| Wall Thickness | Transmural (full), Subendocardial (partial) |
| Age of Infarct | Acute (fresh), Chronic (healed/organized) |
Common Arterial Involvements:
| Artery | Area Affected |
|---|---|
| Left Anterior Descending | Anterior LV, apex, anterior 2/3 septum (40–50%) |
| Right Coronary Artery | Posterior LV, posterior 1/3 septum (30–40%) |
| Left Circumflex Artery | Lateral wall of LV (15–20%) |
MORPHOLOGICAL CHANGES IN MI
Early Changes (within 30 min):
-
Electron Microscopy:
-
Glycogen loss (5 min)
-
Mitochondrial swelling (20–30 min)
-
Sarcolemma disruption
-
-
Biochemical:
-
↑ Lactic acid, ↓ K⁺, ↑ Na⁺, ↑ Ca²⁺ → irreversible injury
-
CLINICAL FEATURES OF ACUTE MI
-
Pain: Severe, crushing, prolonged, radiating; not relieved by rest
-
GI symptoms: Nausea, vomiting, heartburn-like
-
Apprehension: Fear, anxiety
-
Shock signs: Low BP, tachy/bradycardia, cyanosis
-
Oliguria: <20 mL/hr urine
-
Fever: Mild, begins in 24h, lasts a week
-
Pulmonary edema: Due to LV failure – dyspnea, orthopnea
DIAGNOSIS OF ACUTE MI
1. ECG Changes (STEMI):
-
ST Elevation: Key marker
-
T wave inversion
-
Pathologic Q waves
2. Serum Cardiac Markers:
| Marker | Notes |
|---|---|
| CK-MB | Cardiac-specific; rises in 3–12 hrs, peaks in 24 hrs |
| Troponin I/T | Most specific; rises in 3–6 hrs, remains elevated for 7–10 days |
| LDH, Myoglobin | Less specific; earlier rise for myoglobin |
CHRONIC ISCHAEMIC HEART DISEASE (IHD)
Also known as:
-
Ischaemic cardiomyopathy
-
Myocardial fibrosis
Definition:
A long-term condition in which parts of the heart muscle are replaced by fibrous tissue (scarring) due to reduced blood supply (ischaemia). It is commonly seen in elderly patients with a history of angina (chest pain) or heart attacks (MI).
What Happens:
-
The heart slowly becomes weak and less efficient.
-
Patients may develop chronic heart failure (CHF) over years.
-
Sometimes, sudden arrhythmias (abnormal heart rhythms) or another heart attack can occur, leading to sudden death.
CAUSES (Etiopathogenesis):
-
Main cause: Coronary atherosclerosis – gradual narrowing and hardening of coronary arteries due to plaque buildup.
-
Less common causes:
-
Emboli – blood clots blocking arteries
-
Coronary arteritis – inflammation of the coronary arteries
-
Myocarditis – infection or inflammation of heart muscle
-
How Fibrosis Develops:
There are two main theories:
-
Healing of small, unnoticed heart attacks (microinfarcts).
-
Slow degeneration (myocytolysis) of small areas of heart muscle due to poor oxygen supply. These dead muscle cells are removed and replaced by fibroblasts and collagen, forming scar tissue.
MORPHOLOGY (What the heart looks like):
Gross Features (visible to the naked eye):
-
Heart may be normal or enlarged (hypertrophied)
-
Fibrotic (scarred) areas appear as grey-white patches in the heart muscle
-
Previous healed heart attacks may be visible
-
Left heart valves may be thickened and calcified
-
Coronary arteries show severe atherosclerosis
Microscopic Features:
-
Scattered areas of fibrosis, especially around small blood vessels
-
Myocardial cells vary in size; some show myocytolysis (breakdown)
-
Brown atrophy – age-related pigment (lipofuscin) in muscle cells. Lipofuscin is a "wear-and-tear" or "aging pigment". It consists of oxidized lipids, proteins, and metals that accumulate inside lysosomes of long-living cells. It is not harmful but is a sign of cellular aging and oxidative stress.
-
Coronary arteries have atherosclerotic plaques, sometimes with thrombi (clots)
SUDDEN CARDIAC DEATH
Definition:
Unexpected death within 24 hours of developing heart symptoms, most often due to a fatal arrhythmia.
Main Cause:
-
Coronary atherosclerosis (major cause)
-
Other causes include:
-
Coronary artery spasm
-
Calcific aortic stenosis (narrow aortic valve)
-
Myocarditis
-
Hypertrophic cardiomyopathy (thickened heart muscle)
-
Mitral valve prolapse
-
Endocarditis (heart valve infection)
-
Conduction system disorders (inherited or acquired)
-
Mechanism:
-
Usually due to sudden arrhythmias, like:
-
Ventricular asystole (heart stops beating)
-
Ventricular fibrillation (heart quivers instead of pumping)
-
Autopsy Findings:
-
Severe narrowing (≥75%) of one or more major coronary arteries
-
May show:
-
Thrombosis (clot)
-
Plaque rupture or bleeding (plaque hemorrhage)
-
Signs of new or healed infarcts
-












Comments
Post a Comment